Air permeability is an important performance index of precision casting mold shells. The air permeability of the mold shell comes with a great influence on precision castings. For example, it is often found that the insufficient pouring of castings (including incomplete shape and rounded corners), may be caused by poor air permeability of the mold shell. In addition, the air permeability of the mold shell is also an important factor affecting its dewaxing quality.
The Source of Gas
Gases that can be dissolved in metals, mainly hydrogen and oxygen. During the smelting process, the main sources of gas are:
- Furnace gas: during non-vacuum smelting, furnace gas is the main source of gas in metal. In addition to oxygen and nitrogen, furnace gas also contains water vapor, hydrogen, carbon monoxide, carbon dioxide, sulfur dioxide, and hydrocarbons. The composition of furnace gas varies with the fuel used and combustion conditions; For example, the reverberatory furnace or crucible furnace fueled by heavy oil or gas often contains 5-10% water vapor and more hydrogen, carbon monoxide, etc.
- Furnace charge: most of the electrolytic metal surfaces remain residual electrolytes, and most of the returned materials from the processing workshop contain oil, water, emulsion, etc. Most of the foreign wastes come with water corrosives, rust, etc. Especially in the open-air stacking and wet seasons, moisture is adsorbed on the surface of the charge. These will make the metal absorb more hydrogen during the smelting process.
- Refractory: the moisture contained in the refractory can also promote metal inhalation, especially when the new furnace starts production.
- Flux: Many fluxes contain moisture, some of them (such as charcoal, rice bran, etc.) contain adsorbed moisture, and some fluxes (such as borax) contain crystal water. In order to reduce the source of gas in the smelting process, the flux should be dried or dehydrated.
- Operating tools: incomplete preheating of operating tools will also increase the gas content of the metal.
Dissolution Process and Solubility of Gas
Solubility of gas in metal: when the metal is solid, the solubility of gas is very small. With the rise of temperature, the solubility increases slowly, and the solubility increases sharply when it reaches the melting point temperature. Continue to increase the temperature of the molten metal, the gas solubility continues to increase until it reaches the limit. Then it starts to drop, and when the temperature reaches the boiling point of the metal, the gas solubility is almost equal to zero.
Cu+Ni > Cu+Pb > Cu+Ag > Cu+Au > Cu+Sn > Cu+Al
Different alloy elements come with different effects on the solubility of the gas in the alloy. Some elements such as nickel have a greater binding ability with the gas, which increases the solubility of the gas in the alloy. Other elements such as aluminum and tin can reduce the solubility of the gas in the alloy. For copper alloy, the effects of alloy elements on hydrogen solubility are as follows: Cu+Ni > Cu+Pb > Cu+Ag > Cu+Au > Cu+Sn > Cu+Al
Degassing Methods
Gas degassing method: use inert gas (such as N2) or active gas (such as Cl2). The smaller the bubbles, the greater the number, and the more beneficial for degassing. However, due to the high floating speed of bubbles, the time for passing through the melt is short, and the bubbles cannot be evenly distributed in the entire melt, so it is not easy to degas completely by this method. With the decrease of hydrogen content in the melt, the degassing capacity decreases significantly.
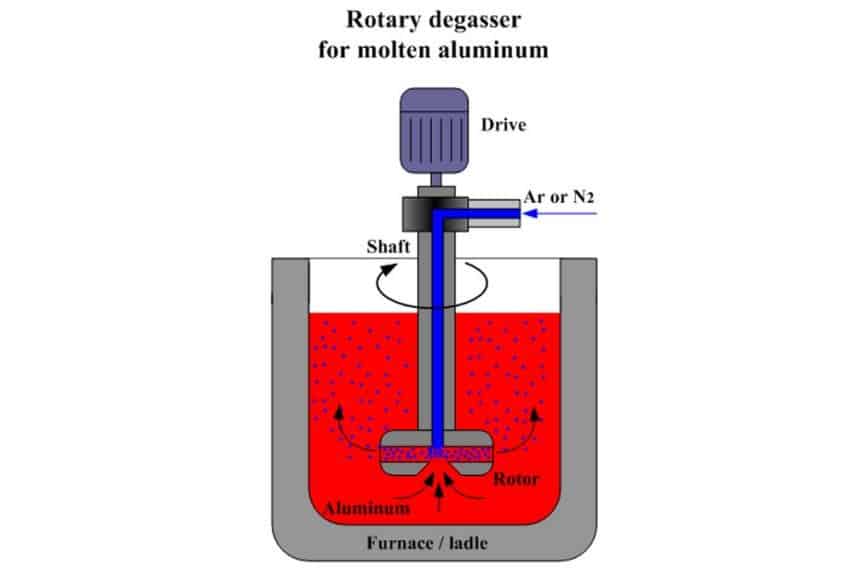
Flux degassing method: flux degassing is the use of thermal decomposition of molten salt or replacement reaction with the metal to generate volatile bubbles that are insoluble in the melt to remove hydrogen. For example, cryolite flux is commonly used for degassing aluminum bronze; fluorite, borax, calcium carbonate, and other fluxes are commonly used for degassing cupronickel and nickel alloys. In order to improve degassing, dry nitrogen can be used to blow the powdered flux into the molten pool, the flux can remove the slag while degassing.

Other degassing methods: condensation degassing, oscillation degassing, DC electrolytic degassing.
